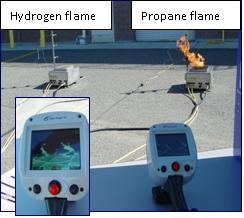
Hydrogen Flames
Hydrogen is flammable at concentrations between 4% and 75% in air, which is a very wide range compared to other common fuels (see Hydrogen Compared to Other Fuels).
- The hydrogen concentration could easily reach the lower flammability limit (4%) if there were a leak in a confined space with no ventilation. An outdoor leak would simply rise quickly and diffuse.
- Hydrogen burns with a pale blue flame that is almost invisible during daylight hours, so fires are almost impossible to see with the naked eye.
- Hydrogen fires have low radiant heat, so you can't sense the presence of a flame until you are very close to it (or even in it).
- Combustion can't occur in a tank or pipe that contains only hydrogen. Oxygen (or air) and an ignition source are required for combustion to occur.
Hydrogen and Propane Flames in Daylight
(Photo courtesy of HAMMER)
Hydrogen and Propane Flames at Night
(Photo courtesy of ImageWorks)
Thermal Imaging Camera in Use
(Photo courtesy of HAMMER)
- Because of these properties, use of a portable flame detector such as a thermal imaging camera may be the best way to detect flames. A simple flammable “indicator” tool such as a broom may also be used to check for hydrogen flames – when the broom comes in contact with the hydrogen flame, it will ignite with a visible flame.
- If flame detection equipment is not available, listen for venting hydrogen and watch for thermal waves indicating the presence of a heat source.
- Note that ignition of venting gaseous hydrogen from vent stacks is common. Vent stacks are designed to handle the ignition safely.
Lesson Learned Reference