Chemical Storage
The term "chemical hydrogen storage" is used to describe storage technologies in which (1) hydrogen is released from a material through a chemical reaction and (2) the hydrogen is restored through a chemical reaction when the material is being recharged. Common reactions involve heating chemical hydrides to release hydrogen and/or reacting chemical hydrides with water or alcohols.
Many of the compounds that are being investigated for chemical hydrogen storage have never been synthesized, so material safety data sheets (MSDSs) do not exist. Chemical hydrides present special hazards, including toxic byproducts, water and air reactivity, pyrophoricity, potential for runaway reactions with gas formation, room-temperature gas emissions, and instabilities, etc. Persons working with these materials should review appropriate MSDSs and reference documents (e.g., Sax's Dangerous Properties of Industrial Materials) to familiarize themselves with the properties and hazards of the individual chemicals that are being used. Additionally, researchers should evaluate the potential interactions and stability of all system components, including reactants, byproducts, and potential compounds formed in liquid, solid, and gaseous states and take appropriate precautions against uncontrolled exposures and reactions. Facilities for conducting experiments with these materials must comprehend these issues.
Best practices for working safely with chemical hydrides should include the following. Keep in mind that these are examples and that there may be other specific precautions that need to be developed for new materials and reactions:
- Hydrogen compounds may react with hydroxides and oxygen-containing organics such as alcohols and ketones to form unstable products, so great care should be taken in selecting and evaluating the safety of these systems. Appropriate risk mitigation strategies should be adopted based on reactivity data and guidance from chemists and those with chemical engineering experience in handling these compounds.
- Generating hydrogen in an exothermic reaction can be potentially explosive. Always perform reactions at laboratory scale until the material properties and reactivity are well understood. Use a chemical reactivity worksheet to determine hazardous combinations of chemicals that can undergo hazardous exothermic reactions
- Gases that are reactive to the atmosphere should be handled using vacuum lines or inert environments to reduce the hazard.
- Solids that are reactive to the atmosphere should be manipulated in an inert glove box to reduce the hazard.
- Air-sensitive liquids and solutions should be handled using safe methods (e.g., under an inert atmosphere using syringe and/or double-ended needle techniques along with appropriate glassware equipped with sleeve stoppers).
- The researcher should evaluate proper pressure-relief system sizing to mitigate any runaway decompositions and ensure that worst-case flammable vapor or gas concentrations remain below combustible limits.
- Work involving hydrogen generation or active boron hydride formation should be performed in an inert atmosphere (e.g., inert glovebox).
- Mixtures or solutions of amine-borane (AB) adducts should be vented until the absence of pressure build-up is assured.
- Researchers should consider the potential presence of diborane (which is highly toxic and pyrophoric) when working with AB and take appropriate precautions.
- All new AB adducts prepared from primary (1°) or secondary (2°) amines should be treated as potentially unstable, pyrophoric, and water-reactive until verified otherwise.
- Ammonium borohydride should be stored at low temperatures, since it is unstable at room temperature.
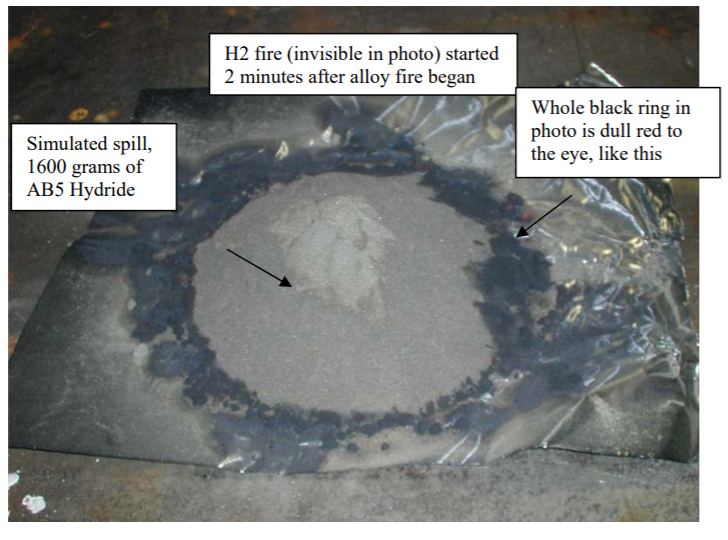
AB5 Metal Hydride Spill Fire
- Hydrazine-bisborane should be treated with extreme caution.
- Alternatives to cold traps should be considered for drying a B-H compound. However, if a cold trap is used, caution should be exercised when liquid nitrogen is used in the cold trap for distilling B-H compounds, as diborane may be formed and collected due to disproportionation. If such a system were opened to air, it could result in an explosion due to the pyrophoricity of diborane. To mitigate this hazard, small amounts of methanol could be added to the trap before distilling the B-H compound to prevent the diborane accumulation.
- Provide vents to accommodate hydrogen generation rates and be sure to vent hydrogen out of building. Provide hydrogen sensors to detect inadvertent hydrogen releases into building.
Incident example: Uncontrolled hydrogen generation from an unvented silicon hydride emulsion vessel leads to a hydrogen release into building and an explosion with four fatalities.
CSB Factual Update, Explosion and Fire at AB Specialty Silicones Facility, May 3, 2019, CSB Report 2019-02-IL