Hydrogen Compared To Other Fuels
Like gasoline or natural gas, hydrogen is a fuel that must be handled properly. It can be used as safely as other fuels when guidelines are followed.
Produced by the U.S. Department of Energy, the Hydrogen Analysis Resource Center provides useful data on hydrogen properties, including:
- Chemical characteristics of hydrogen (e.g., density, flammability range, boiling point characteristics, heating values)
- Comparisons of the characteristics of hydrogen and many other fuels.
Bar charts presented in the following sections compare some key properties of hydrogen with those of natural gas, propane, and gasoline vapor.
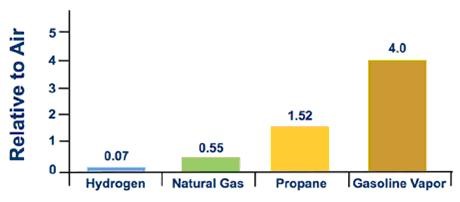
Figure 1. Relative Vapor Density
Hydrogen is a very small molecule with low viscosity, and therefore prone to leakage. In a confined space, leaking hydrogen can accumulate and reach a flammable concentration. Any gas other than oxygen is an asphyxiant in sufficient concentrations. In a closed environment, leaks of any size are a concern, since hydrogen is impossible for human senses to detect and can ignite over a wide range of concentrations in air, as discussed in the section below. Proper ventilation and the use of detection sensors can mitigate these hazards.
Hydrogen has a high energy content by weight, but not by volume, which is a particular challenge for storage. In order to store sufficient quantities of hydrogen gas, it's compressed and stored at high pressures. For safety, hydrogen tanks are equipped with pressure relief devices that will prevent the pressures in the tanks from becoming too high.
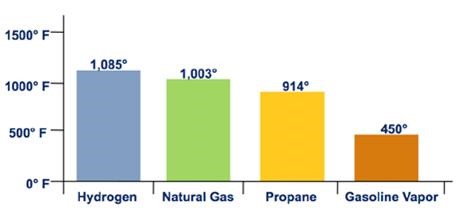
Figure 2. Auto Ignition Temperature
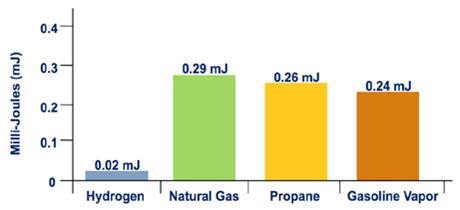
Hydrogen's flammability range (between 4% and 75% in air) is very wide compared to other fuels, as shown in Figure 3. Under the optimal combustion condition (a 29% hydrogen-to-air volume ratio), the energy required to initiate hydrogen combustion is much lower than that required for other common fuels (e.g., a small spark will ignite it), as shown in Figure 4. But at low concentrations of hydrogen in air, the energy required to initiate combustion is similar to that of other fuels.
Figure 3. Flammability Range
Figure 4. Minimum Ignition Energy
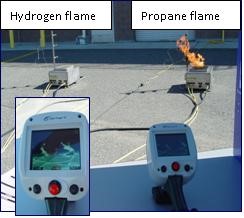
Hydrogen burns with a pale blue flame that is nearly invisible in daylight, so it is almost impossible to detect by the human senses (see Hydrogen Flame Characteristics video under References in the right column of this page). Impurities such as sodium from ocean air or other burning materials will introduce color to the hydrogen flame. Both hydrogen and flame detectors are almost always installed with hydrogen systems to quickly identify any leak and minimize the potential for undetected flames. Compared to the propane flame (right) in Figure 5, the hydrogen flame (left) is almost invisible, but it can be seen with the thermal imaging camera shown in the foreground. At night, hydrogen flames are visible, as shown in Figure 6.
Figure 5 - Hydrogen and Propane Flames in Daylight
(Photo courtesy of HAMMER)
Figure 6 - Hydrogen and Propane Flames at Night
(Photo courtesy of ImageWorks)
In addition, hydrogen flames radiate little infrared (IR) heat, but substantial ultraviolet (UV) radiation. This means that when someone is very close to a hydrogen flame, there is little sensation of heat, making inadvertent contact with the flame a significant concern. UV overexposure is also a concern, since it can result in sunburn-like effects.
If a large hydrogen cloud comes into contact with an ignition source, ignition will likely result in the flame flashing back to the source of the hydrogen. In open spaces with no confinement, flames will propagate through a flammable hydrogen-air cloud at several meters per second, and even more rapidly if the cloud is above ambient temperature. The result is a rapid release of heat, but little overpressure, and the combustion product is steam. It should be noted that hydrogen combustion is more rapid than combustion of other fuels. A hydrogen cloud will burn within seconds, and all of the energy of the cloud will be released.
However, a hydrogen gas mixtures ignited in a confined space can generate pressures high enough to rupture equipment, exploding buildings and throw shrapnel. So, keeping hydrogen equipment and piping outdoors is an inherent safety advantage.
A large leak in a pressurized [>1400 kPa (>200 psi)] hydrogen system will result in a jet that may extend for some meters. If ignited, the jet flame can cause serious damage to anything it encounters. This is the reason for establishing separation distances between hydrogen systems and objects that can be harmed by a jet flame.
References
Basic Hydrogen Properties Chart
GA G-5: Hydrogen
CGA H-4: Terminology Associated with Hydrogen Fuel Technologies
B. Lewis and G. von Elbe, Combustion, Flames and Explosions of Gases, 3rd ed., Academic Press, Orlando, 1987, pg. 717.
G-095A-2017: ANSI/AIAA Guide to Safety of Hydrogen and Hydrogen Systems.
CRC Recent Developments in Hydrogen Technology, Volume II. Editors K. D. Williamson, Jr. and Frederick J. Edeskuty, CRC Press, Inc. Boca Raton, Florida 1986. See Chapter 2, “Hydrogen Behavior and Nuclear Safety” by Marshall Berman, p. 35.
(mov, 7.4 mb), filmed by ImageWorks for DOE.
Babrauskas, Vytenis. "Ignition Handbook" Fire Science Publishers, Issaquah, WA.
J. Hord, Is Hydrogen Safe? National Bureau of Standards (NBS) Technical Note 690, October 1976.
NASA White Sands Test Facility Hydrogen Plume Tests video
(mov, 7.4mb)
F.J. Edeskuty and W.F. Stewart, Safety in the Handling of Cryogenic Fluids, Plenum Press, New York, 1996, pg. 102.
R. D. McCarty, J. Hord and H. M. Roder, NBS Monograph 168, Selected Properties of Hydrogen (Engineering Design Data), Center for Chemical Engineering, National Engineering Laboratory, National Bureau of Standards, Boulder, CO 80303, published by U.S. Department of Commerce, U.S. Government Printing Office, Washington, D.C. 1981.
NFPA 2, Hydrogen Technologies Code