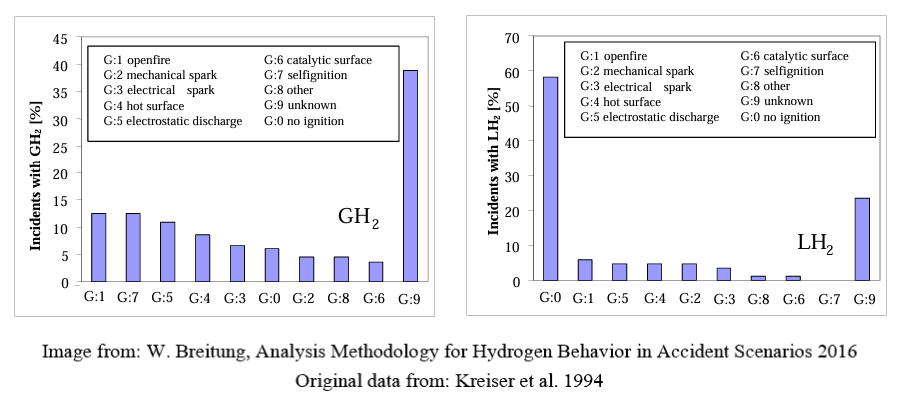
It is not possible to define ignition potential by just velocity without more data (i.e. pressure, materials involved, direction of impact). Due to the multiple methods of developing an ignition source (friction, impact, electrical charge) and the low ignition energy, it is assumed that hydrogen in the air will ignite (between 4 -74%), as it does 30-40% of the time with no known ignition source (see GH2 chart below). Therefore, to try and manage impingement by velocity as an ignition source is not a practical method to assure no ignition.
Other Information:
The ignition energy of hydrogen is .02 millijoules. By definition, a joule is equal to the kinetic
energy of a kilogram mass moving at the speed of one meter per second.
From “Mechanical Sparks as an Ignition Source of Gas and Dust Explosions” from The Italian Association of Chemical Engineering Online:
Mechanical sparks are small particles, which due to the impact between two objects are torn loose from the surface of one of the two colliding objects. The kinetic energy is turned into heat and deformation work. Mechanical spark generation is dependent on the pressure with which the one object is working against the other, the relative speed between the objects, the friction coefficient and the hardness of the materials involved.
Extrapolation of the experimental results using a model it could be shown that incendive hot surfaces can be generated also at relative speeds of < 1 m/s.
Additionally, tests were performed using a file traveling at 1m/s against a metal surface, and the ignition of hydrogen over many concentrations was observed.


