Handling Cryogenic Liquid
Installations which use large amounts of hydrogen may be best supplied with cryogenic liquid hydrogen (LH2) as it is easier to transport and deliver than large volumes of compressed gas. Typical large-scale hydrogen supply systems will include a permanent installation including cryogenic storage, vaporization, and compression equipment owned and maintained by the hydrogen supplier. In some cases small amounts of LH2 may be needed for laboratory or experimental work. In such cases it is extremely important that anyone using LH2 is specifically trained to handle it safely.
Anyone working with hydrogen needs to understand the basic characteristics of hydrogen gas (see Hydrogen Properties and Leak Detection Considerations).
Additional considerations for cryogenic liquid hydrogen include:
- LH2 has an extremely low boiling point [-253°C (-423°F)] and can cause severe frostbite and hypothermia.
- All gases except helium are solid at LH2 temperatures.
- When stored properly, liquid hydrogen will warm and vaporize slowly. The gas thus generated must be used or vented. Boil off rates less than 1% of full storage capacity per day can be achieved. Refrigeration to condense evaporated hydrogen is usually not an economical option.
- The continuing evaporation of liquid hydrogen increases pressure and results in pressure failure of the component if not properly released. Therefore any LH2 storage container must include a properly designed pressure relief system intended specifically for LH2 and a properly designed vent system (see Venting). It’s important to note that periodic venting of excess pressure from LH2 boil-off is a normal part of the operation of an LH2 system.
- If a liquid hydrogen leak or spill occurs, a hydrogen gas cloud could flow horizontally for some distance or even downward until the gas warms. The warmed gas will mix with air, creating flammable clouds that, if ignited, will result in explosions or fires. (Fire codes refer to an LH2 spill as a “boiling liquid, explosive vapor” situation.)
- Ice formation from condensing atmospheric moisture on the outside of valves can prevent the valves from being operated.
- Ice formation on the inside of vent lines can block the vent flow, resulting in equipment failure by overpressure.
- Condensed air on uninsulated LH2 equipment could result in oxygen enrichment and explosive conditions near a liquid hydrogen system.
- The required separation distances for liquid hydrogen facilities are generally larger than required for gas systems. See, for example, NFPA 2.
Liquid hydrogen leaks are easier to detect than leaks of compressed gas for the following reasons:
- Because of the cold temperature, a leak from a liquid hydrogen system may create frost or ice crystals on the outside of affected components.
- Hydrogen gas formed by boiling LH2 is heavier than air and will stay near the ground until it warms. In the process, it will condense water vapor from the air, creating a cold fog. Even in dry climates, a liquid hydrogen spill will create a white cloud of condensed water vapor (see photo below). The cloud will remain localized and may appear to move horizontally or even downward. As the hydrogen warms, it will dissipate and rise quickly, carrying the water vapor upward as it rises.

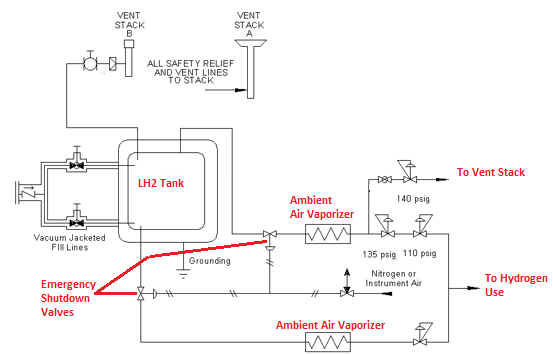
Liquid Hydrogen systems typically consist of the following components:
- Liquid hydrogen storage vessel – Double wall vessel design, like a thermos bottle
- Vacuum jacketed liquid transfer lines
- Vacuum pump to maintain the vacuum
- Heat exchangers – typically ambient air vaporizers. These convert the liquid to gas and warm the gas to near ambient air temperature
- Control manifolds that keep the pressure constant and shutoff the hydrogen flow when it gets too cold
- Compressors are needed for pressures above the tank operating pressure, typically around 850 kPa (~123 psi)
Controls and safety features include:
- Helium purge system to remove the air/nitrogen from liquid transfer lines prior to filling
- Emergency shutdown valves on the liquid and gas product withdrawal lines
- Pressure relief devices
- Pressure controls that are used to vent excess hydrogen at pressures lower than the set pressure for the relief devices.
The large temperature difference between ambient and cryogenic conditions results in significant thermal contraction of most materials, which must be accommodated in designs for cryogenic service.
Air Products Safetygram for Liquid Hydrogen
CGA P-12, Safe Handling of Cryogenic Liquids